5 Minutes
A small clinical trial in the United States suggests two repurposed drugs — a diabetes medication and an intranasal insulin spray — may safely improve markers of brain health in people at risk of Alzheimer's disease. The study focused on metabolic and neurovascular measures linked to early cognitive decline and produced encouraging signals that merit larger, longer trials.
Why researchers targeted metabolism and blood flow
Alzheimer's and related dementias are biologically complex, and treating them will likely require several complementary strategies. Rather than attacking late-stage hallmarks such as large amyloid plaques, the trial tested treatments that influence cellular metabolism, inflammation and brain circulation — upstream processes that can make neurons more resilient.
The two interventions were empagliflozin, an established diabetes and cardiovascular drug, and a nasal insulin spray designed to deliver insulin directly to the brain. Empagliflozin alters glucose and sodium handling in the body, which reduces cellular stress and inflammation and can improve energy efficiency. Intranasal insulin aims to counter brain insulin resistance — a factor increasingly implicated in cognitive decline — while supporting neuronal health and immune function in the central nervous system.
Trial design and who took part
The phase II safety trial enrolled 47 adults aged 55–85; 42 completed the four-week protocol. Participants included people with mild cognitive impairment (MCI) or mild dementia, as well as cognitively normal volunteers who showed molecular signs of Alzheimer's disease on biomarker testing. Subjects were randomized to one of four arms: empagliflozin alone, insulin spray alone, both treatments together, or placebo.
Because the primary aim was to evaluate safety and tolerability over a short period, the study was not powered to detect definitive efficacy. Still, researchers collected a broad set of biomarkers and imaging outcomes — cerebrospinal fluid (CSF) assays, cognitive tests, white matter connectivity on MRI, cerebral blood flow measures, and cholesterol and inflammatory markers — to look for early trends.
Key findings: signals in tau, cognition and circulation
Although differences between groups did not reach statistical significance given the small sample and brief treatment window, several notable patterns emerged. People taking empagliflozin alone showed reductions in CSF tau levels — a protein that can aggregate into toxic tangles in Alzheimer's brains — alongside improvements in blood flow and certain lipid markers linked to dementia risk.
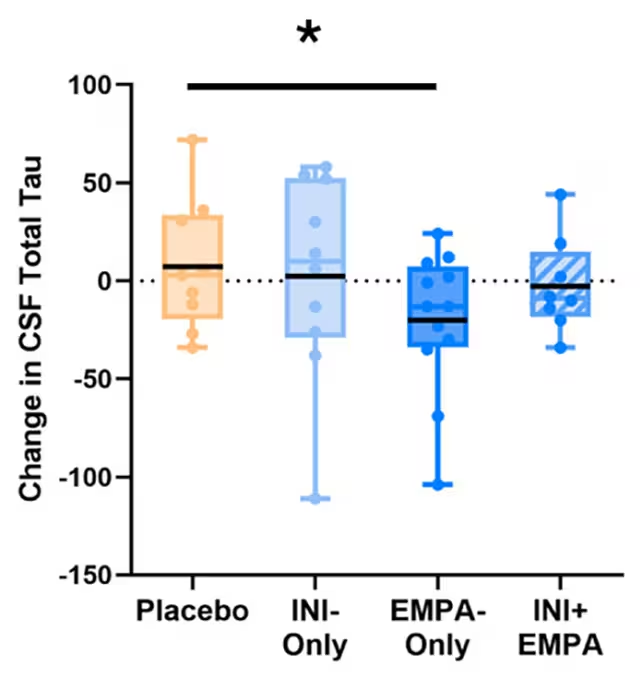
One of the benefits seen with empagliflozin (EMPA-Only) was signs of less tau build-up in cerebrospinal fluid (CSF). (Erichsen et al., A&D, 2025)
Those who received the intranasal insulin recorded better scores on memory and executive function tests over the short trial period. Brain imaging from these participants also revealed improved white matter connectivity and enhanced perfusion in regions tied to memory processing, consistent with prior studies that connect insulin signaling to neurovascular health.
How these drugs could work together
The trial supports a model in which metabolic modulation and targeted neuroendocrine support have complementary effects. Empagliflozin appears to reduce systemic inflammation and cellular stress, potentially slowing processes that promote tau pathology. Intranasal insulin seems to directly support neuronal metabolism, synaptic function and immune regulation in the brain. Together, they may strike a balance between boosting protective immune responses and avoiding harmful neuroinflammation.
Safety and limitations
No harmful side effects were reported in this short study, and both treatments were tolerated across age groups and clinical subtypes. But the authors emphasize the trial’s limitations: small sample size, brief exposure (four weeks), and the exploratory nature of multiple biomarker endpoints. Larger randomized trials of longer duration are required to confirm whether these early biomarker and cognitive signals translate into clinically meaningful slowing of disease progression.
Next steps: scaling up and combination therapy
Investigators plan to test these therapies in larger cohorts with longer follow-up to quantify effects on cognition, functional decline, and validated biomarkers of Alzheimer's disease. The prospect of repurposing an approved diabetes drug and a targeted insulin-delivery device is appealing because both have established safety profiles and could reach patients faster if efficacy is shown in later-stage trials.
Expert Insight
Dr. Maria Alvarez, a neurologist and clinical researcher not involved in the study, commented: "This trial is an example of pragmatic, mechanism-driven research. Targeting metabolism and vascular health early makes physiological sense. The signals are modest but consistent enough to justify larger trials that can test real-world benefit."
Overall, the findings highlight metabolism and neurovascular health as actionable fronts in the fight against Alzheimer’s, and they open a path toward combination therapies that intervene earlier in the disease cascade.
Source: sciencealert
Comments
Marius
Wow nasal insulin + empagliflozin? kinda hopeful here, really want bigger trials, longer followup pls!!! curious but nervous.
atomwave
hmm interesting but 4 weeks? tiny sample, are those tau drops meaningful or just noise... if that's real then worth trying longer

Leave a Comment