5 Minutes
Scientists are turning to a surprising class of two-dimensional compounds—MXenes—to rethink how we produce fuels and essential chemicals. These atom-thin materials, built from transition metal carbides and nitrides, offer tunable chemistry and promising performance as electrocatalysts that could lead to cleaner ammonia production and more efficient renewable-energy systems.
Why MXenes are grabbing researchers' attention
MXenes are thin, layered materials whose composition can be tuned at the atomic level. That flexibility is crucial: by swapping elements in their lattice—such as introducing nitrogen in place of carbon—researchers can alter surface reactivity, electronic behavior, and vibrational modes. In practical terms, MXenes can be engineered to favor specific chemical reactions, including the electrochemical conversion of atmospheric nitrogen into ammonia.
Producing ammonia today is energy intensive, relying on the century-old Haber-Bosch process that consumes fossil fuels and emits large amounts of CO2. Electrocatalytic approaches driven by renewable electricity promise a cleaner path, but they need catalysts that are efficient, durable, and inexpensive. MXenes, especially nitride varieties, are emerging as viable alternatives to traditional, costly catalysts based on precious metals.
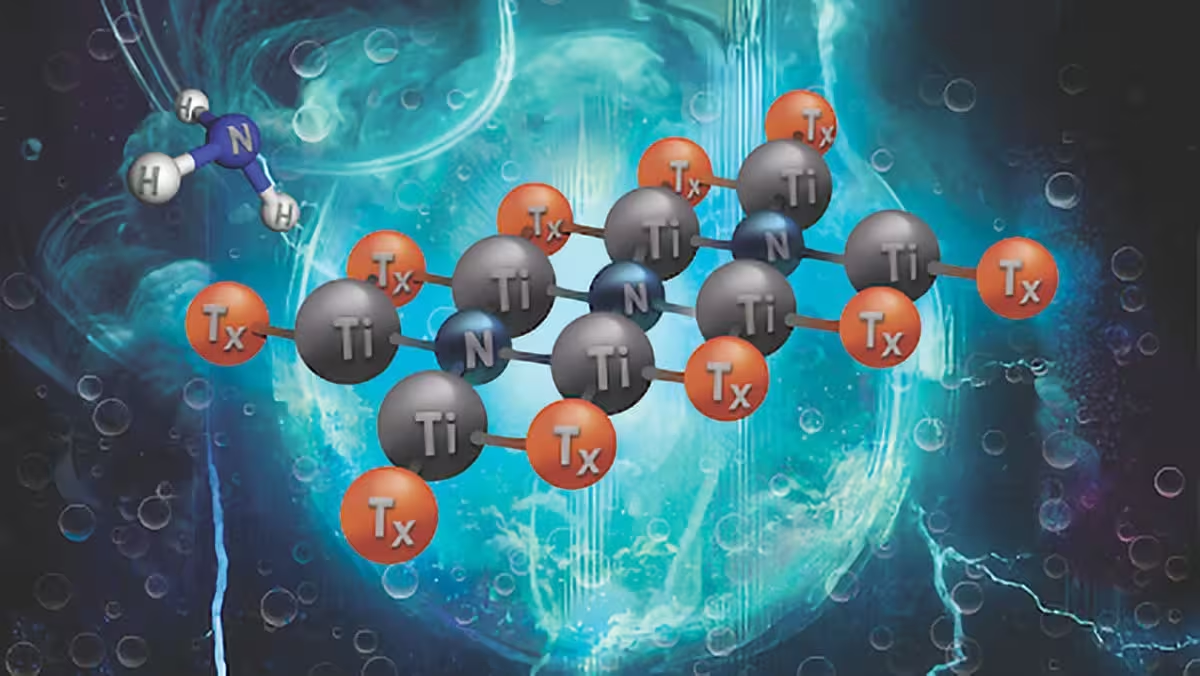
MXenes (pictured above) are a new class of two-dimensional materials composed of transition metal carbides and nitrides with highly tunable electrical and chemical properties. Their remarkable versatility and potential in renewable energy, catalysis, and electronics have led some scientists to describe them as a wonder material. Credit: Dr. Abdoulaye Djire/Texas A&M University
Turning air into fertilizer: the promise for ammonia synthesis
Ammonia is central to fertilizer production and also a promising energy carrier. The team led by Drs. Abdoulaye Djire and Perla Balbuena at Texas A&M University, with Ph.D. candidate Ray Yoo, has been studying how MXenes can catalyze electrochemical nitrogen reduction reactions (NRR). Their work, published in the Journal of the American Chemical Society, suggests that nitride MXenes can significantly improve electrocatalytic performance compared with carbide counterparts.
Key to this improvement is the behavior of lattice nitrogen atoms. By enabling protonation and replenishment of lattice nitrogen under electrochemical conditions, MXenes can participate dynamically in the reaction pathway. That means the material doesn’t simply serve as a passive surface; its atomic makeup can actively engage in binding and transforming nitrogen molecules into ammonia.
Computational and spectroscopic evidence: understanding the mechanism
The research combines first-principles computational modeling with laboratory spectroscopy. Hao-En Lai, a Ph.D. student in Dr. Balbuena’s group, used atomistic simulations to quantify how solvents and reaction intermediates alter MXene surface vibrational modes. Those changes matter: vibrational properties influence how molecules adsorb and react at the surface.
Experimentally, Djire’s group probed titanium nitride MXenes using Raman spectroscopy, a non-destructive technique that maps vibrational signatures of a material. Raman spectra revealed changes tied to lattice nitrogen reactivity, offering a direct spectroscopic handle on how MXenes participate in electrocatalytic nitrogen reduction.
"Our goal is to move beyond the idea that catalyst performance depends only on the metal element," Djire explained. Instead, the team emphasizes the full structural context—lattice atoms, vibrational dynamics, and solvent interactions—when evaluating catalytic function.
What this means for renewable energy and industry
If MXenes can be reliably tuned to drive ammonia synthesis at high efficiency and low overpotential, the implications are wide. Cleaner ammonia production could decarbonize fertilizer manufacturing and enable ammonia to substitute for fossil fuels in certain energy-storage and transportation roles. MXenes’ relative abundance and tunability could also reduce dependence on scarce precious metals in a range of electrocatalysts.
Yet challenges remain. Translating atomic-scale insights into scalable electrodes requires addressing material stability, mass transport in real devices, and long-term reproducibility. Researchers must also optimize how MXenes interact with electrolytes and integrate them into practical reactor designs.
Expert Insight
"MXenes give us an unprecedented level of control over the surface chemistry of catalysts," says Dr. Elena Marquez, a materials scientist not involved in the study. "By combining theory and spectroscopy, teams like Djire’s can identify design rules that guide synthesis toward real-world devices. The next steps will be engineering stability and integrating these materials into prototype electrolyzers."
As the field progresses, MXenes could become a cornerstone of renewable-driven chemical production—bridging fundamental materials science with urgent climate and agricultural needs.
Research teams continue to refine models, expand experimental characterization, and test MXenes under practical operating conditions. With multidisciplinary effort, these two-dimensional materials may help rewrite how we produce vital chemicals and use renewable electricity to power a low-carbon economy.
Source: scitechdaily
Comments
DaNix
Hmm is this even scalable? lab tests ok, real plants? sounds promising but skeptical, needs ton of real world data
labcore
Whoa, MXenes might actually change fertilizer game? if they’re stable, huge deal! curious about cost though...


Leave a Comment