2 Minutes
Scientists are experimenting with engineered bacteria that seek out and attack tumors — a bold idea that could transform cancer treatment from fixed pills into adaptive living therapies. Early human trials show promise, but safety, dosing and containment remain critical hurdles before these “living medicines” can enter routine clinical use.
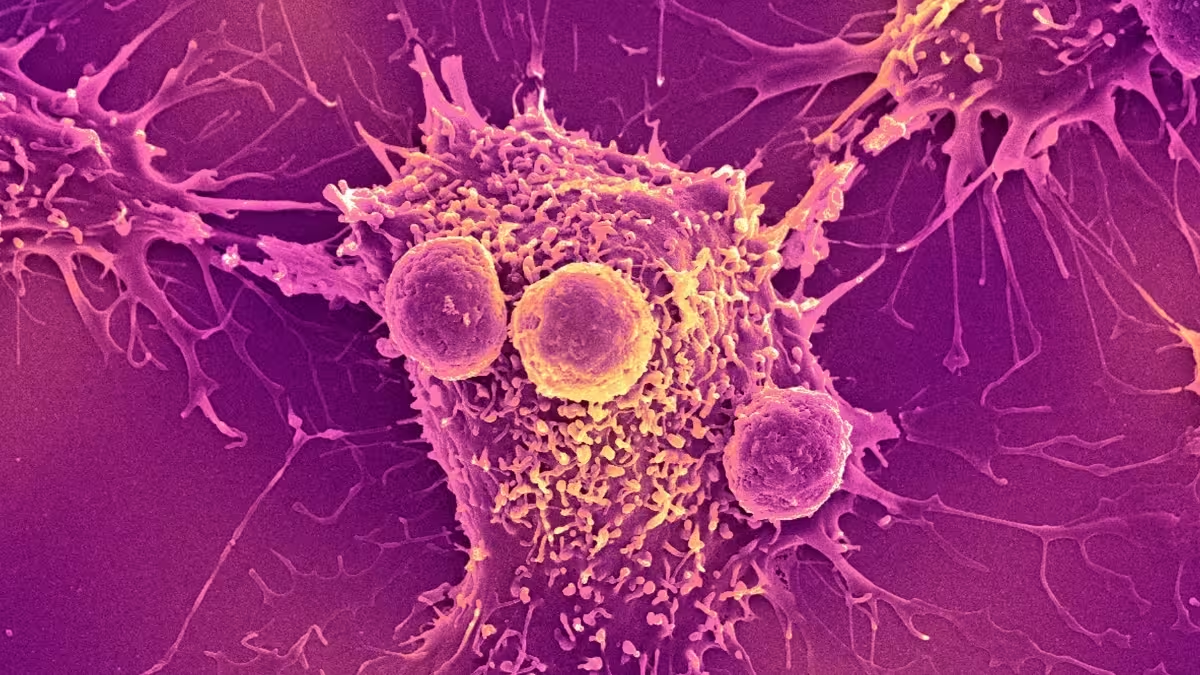
Why bacteria? A new kind of cancer fighter
Certain bacteria naturally home to low-oxygen, nutrient-poor regions inside solid tumors. Bioengineers are harnessing that behavior: microbes can be reprogrammed to deliver anti-cancer payloads, stimulate the immune system, or report tumor status. Imagine a microscopic courier that multiplies at the disease site and adapts its response — that’s the appeal of bacterial therapies.
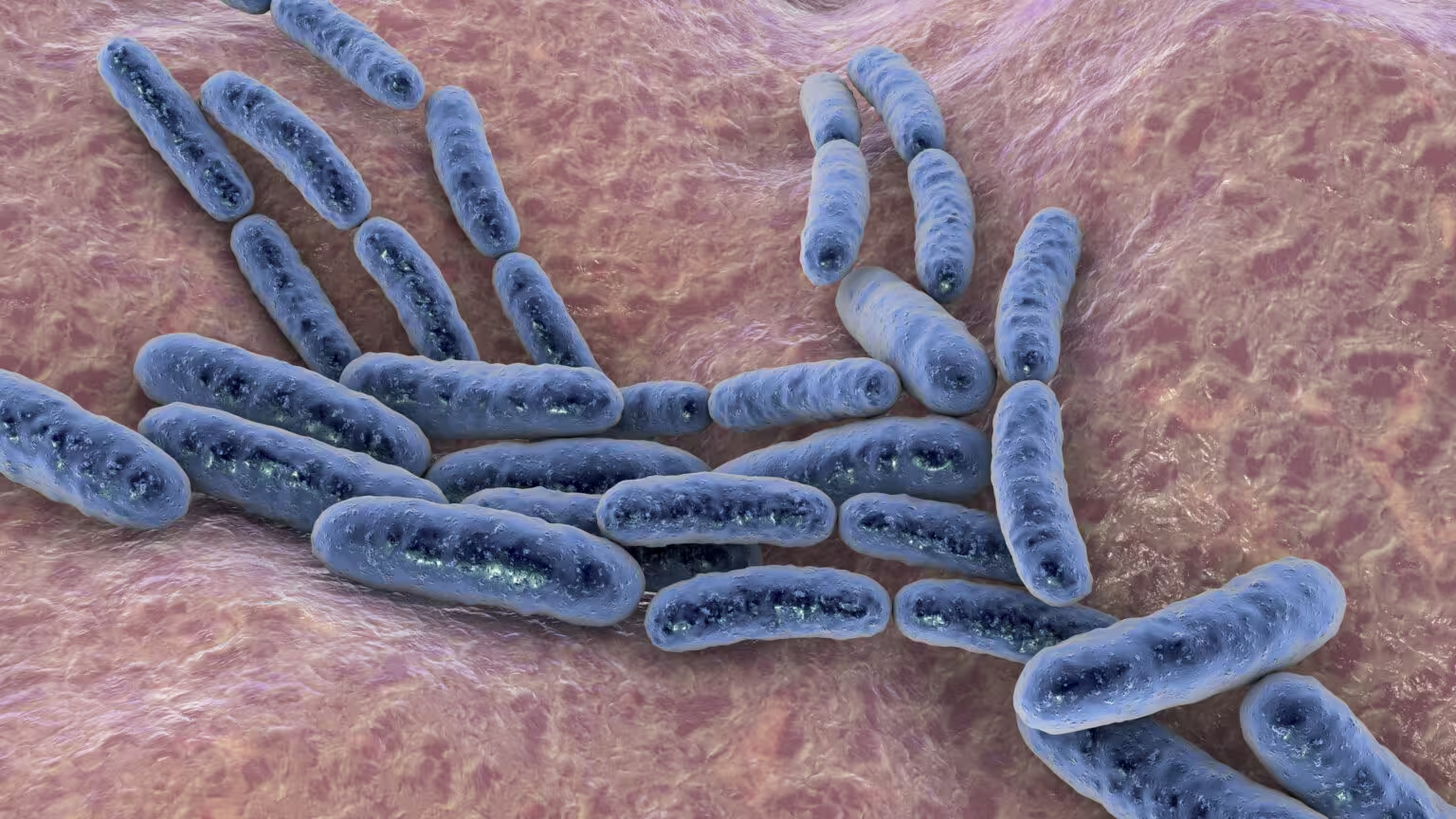
Safety first: dose, infection risk, and immune reaction
Early-phase trials indicate these approaches can be tolerated, but determining the right dose is a delicate balancing act. Too little, and the therapy is ineffective; too much, and patients risk uncontrolled infection or harmful inflammation. Even strains altered to reduce virulence can evolve inside the body, so continuous monitoring is essential.
Biocontainment: engineered safeguards to limit risk
To reduce danger, researchers are developing biocontainment strategies — genetic switches and kill-switches that limit bacterial growth, restrict activity to tumors, or trigger self-destruction after a defined task. These engineered safeguards aim to prevent spread to healthy tissues and curb the chance of unintended evolution.
The regulatory road and future prospects
Beyond laboratory success, living medicines must clear rigorous clinical trials and regulatory review. If they do, we could see a fundamental shift: moving from static drugs to adaptive biological systems that evolve with disease dynamics. That transition promises targeted, potentially more effective cancer care — but only if safety and oversight keep pace with innovation.
Source: sciencealert

Leave a Comment