4 Minutes
Researchers in Spain have pinpointed a small group of amygdala neurons that can trigger — and, when rebalanced, reverse — anxiety-like behavior in mice. By targeting the gene GRIK4 and its protein product GluK4, the team restored normal social behavior and reduced depressive signs in animals bred to show anxiety, opening a precise path for future therapies.
How a few cells change emotional balance
The study, led by the Spanish National Research Council and Miguel Hernández University of Elche (CSIC-UMH) and published in iScience (2025), focused on the basolateral amygdala (BLA) — a brain hub for fear, decision-making and emotional memory. Instead of looking for whole‑brain changes, the researchers tracked activity in a specific neuronal population within the BLA and found that an imbalance there was enough to produce pathological anxiety and social deficits.
When the GRIK4 gene was overexpressed, production of the GluK4 protein increased. Mice with elevated GluK4 avoided open areas, withdrew socially and showed depression-like traits. They also struggled with object-recognition tasks, hinting at wider circuit effects beyond the BLA.
Gene editing calms anxious brains in mice
To correct these behaviors, the team used targeted gene-editing tools to remove extra copies of GRIK4 in the BLA, lowering GluK4 levels. The result was striking: anxiety-like and social-deficit behaviors disappeared, returning treated mice to more typical exploration and interaction patterns. As neuroscientist Álvaro García put it, 'That simple adjustment was enough to reverse anxiety-related and social deficit behaviors, which is remarkable.'
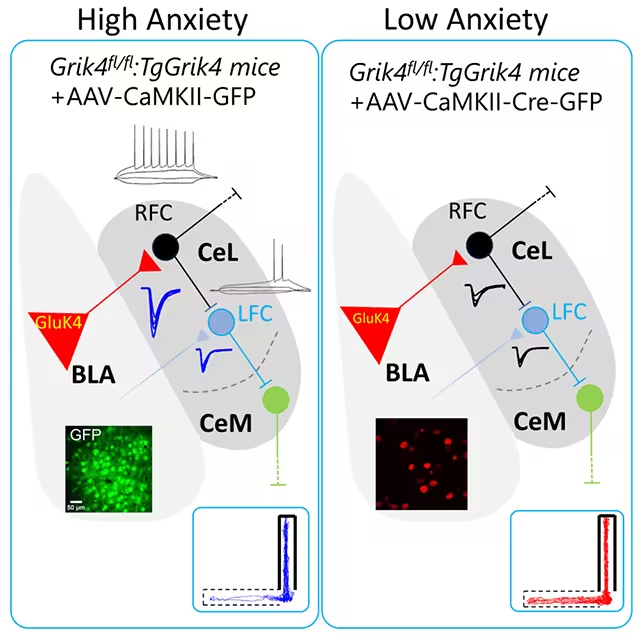
Importantly, the same intervention worked in non‑engineered mice with naturally higher anxiety, demonstrating the approach is effective even outside genetically engineered models. However, treated mice continued to show object-recognition problems, indicating that some cognitive impacts of anxiety involve other brain regions the treatment did not address.
Why this matters — and what we still don't know
Identifying a specific neuronal population whose activity alone can cause pathological anxiety reframes how scientists might treat affective disorders. Targeting discrete circuits offers the prospect of localized therapies with fewer side effects than broad-acting drugs that affect the whole brain.
Still, translation to humans remains speculative. Mice are valuable preclinical models, but human brains are more complex. 'We already knew the amygdala was involved in anxiety and fear, but now we've identified a specific population of neurons whose imbalanced activity alone is sufficient to trigger pathological behaviors,' said Juan Merma, a co-author on the paper. Another researcher, Lerma, noted that targeting these circuits could become 'an effective and more localized strategy to treat affective disorders.'
Future directions and therapeutic prospects
Next steps include mapping how GluK4 dysregulation alters connected circuits beyond the BLA and testing delivery methods that could safely modulate GRIK4 in larger animals. Techniques similar to those used in this study — precise gene modulation in targeted brain regions — might eventually be adapted for humans, either via gene therapy, viral vectors or highly selective pharmacology aimed at GluK4-containing receptors.
For now, the work offers a clearer mechanistic picture: small neuronal imbalances in the amygdala can create outsized emotional effects, and correcting those imbalances can reverse many but not all symptoms. That distinction will guide both basic research and the design of next‑generation treatments for anxiety disorders.
Source: sciencealert

Leave a Comment