8 Minutes
Imagine a counterintuitive twist in cancer biology: very old animals showing less cancer, not more. New research from Stanford University finds that mice in advanced age develop fewer and smaller lung tumors than younger animals with the same cancer-causing mutations. The results challenge the tidy assumption that cancer risk simply climbs unchecked with time and point to age-related changes that might, paradoxically, suppress tumor formation.
Researchers behind the study say the findings help explain a long-observed human pattern: cancer incidence increases into late adulthood but often levels off or declines in the very elderly. The Stanford team used genetically engineered mice and careful molecular analyses to explore which aspects of aging might curb cancer and how specific mutations behave differently across the lifespan.
A surprising pattern in old mice: fewer, smaller lung tumors
The Stanford group compared tumor formation in two age cohorts of laboratory mice: 'young' adults (four to six months old) and animals reaching advanced age (20 to 21 months, roughly the human equivalent of extreme old age). To trigger lung cancer, the researchers used a targeted inhaled gene-delivery system that produces fluorescently labeled tumors, allowing precise imaging and measurement.
After inducing cancer and waiting 15 weeks, the contrast was stark. Young mice developed roughly three times the tumor burden measured in lung weight and fluorescent area, and they had about three times as many individual tumors. The tumors in young animals were also larger and faster-growing. In short, by every metric used, younger mice showed more aggressive disease.
These results mirror epidemiological observations in humans: cancer rates climb through mid- and late-life but often plateau or dip after roughly age 85. Until now, scientists debated whether that drop reflected fewer screenings and underdiagnosis in the very old or biological factors that reduce tumor emergence. The new mouse data suggest a genuine, age-linked biological effect that suppresses tumor initiation and growth.
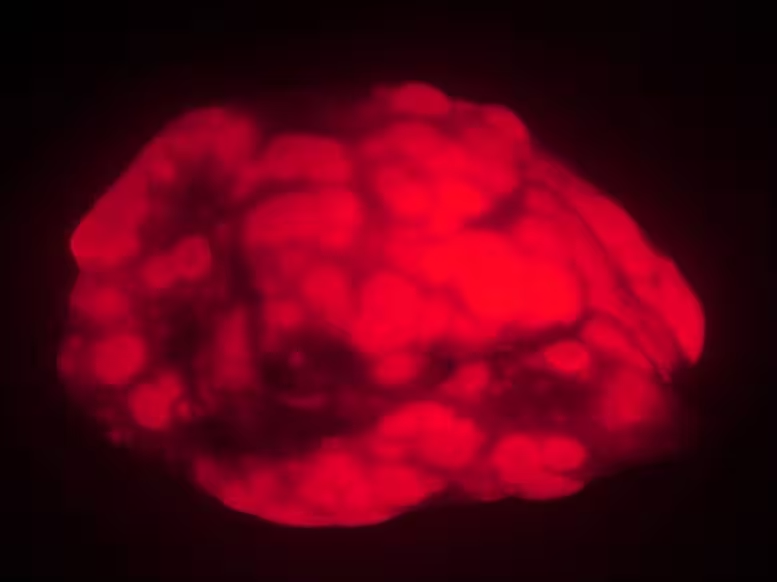
Old mice develop fewer and smaller lung tumors (red) than younger animals in a cancer model. The findings suggest that, in very old animals or humans, the aging process suppresses cancer formation.
What changes with age could block cancer?
At first glance, aging seems to favor cancer: each cell division risks DNA damage, and many hallmarks of aging—genomic instability, altered DNA methylation patterns, and mitochondrial DNA rearrangements—look like fertile ground for malignant transformation. For much of adulthood, that accumulation of damage does correlate with rising cancer incidence.
Yet the Stanford team argues that some age-associated changes may actually become hostile to neoplasia. The study examined how inactivating a panel of 25 known tumor-suppressor genes affected tumor formation in young versus old mice. Tumor suppressors are genes whose protein products normally restrain uncontrolled cell growth; when these genes are lost or disabled, cancers more readily emerge.
Although turning off many tumor-suppressor genes increased tumors in both age groups, the magnitude of that effect was almost always larger in young mice. In other words, the same genetic blow produced a worse outcome in youth. One gene stood out: PTEN. Inactivation of PTEN had a markedly stronger tumor-promoting effect in young animals than in old ones, implying that the biological context of an aged tissue alters how a major cancer pathway operates.
To probe mechanisms, the researchers sequenced gene expression patterns in cancer cells from old versus young mice and from tumors with or without PTEN. Unexpectedly, even rapidly dividing cancer cells isolated from old animals retained molecular signatures associated with aging. These aging-related transcriptional patterns were diminished when PTEN was inactivated, and PTEN-deficient cancer cells in old mice resembled the gene-expression profile of young tumors—an intriguing sign that some aging features can be erased when key pathways are disrupted.
A brief note on technical context
The study used fluorescent tagging to quantify tumor burden, controlled gene inactivation tools to test tumor suppressor effects, and RNA profiling to examine how aging signatures persist in transformed cells. The experimental design required a long timeline—researchers waited nearly two years for mice to reach advanced age, highlighting why aging effects are hard to study experimentally.
Why the findings matter for cancer research and treatment
The practical takeaways are substantial. First, commonly used cancer models—often based on young adult mice—may not capture critical age-dependent biology. That can skew preclinical testing of targeted therapies, which might behave differently in older tissues where the molecular landscape has shifted.
Second, the study suggests that aging is not universally tumor-promoting. Some aspects of the aged microenvironment or cell-intrinsic changes may oppose tumor initiation or slow progression. Understanding those protective mechanisms could reveal new therapeutic strategies: if aging rewires tissue to resist cancer, can that wiring be mimicked therapeutically?
Third, the differential effect of PTEN inactivation warns that specific driver mutations and their targeted treatments may perform differently by patient age. Precision oncology typically matches drugs to mutational profiles—but age-related context may modulate the potency of those mutations and therefore the efficacy of treatments aimed at them.
Monte Winslow, associate professor of genetics and pathology and a senior author on the paper, says the results demand a rethink of model selection for translational research. Dmitri Petrov, a senior coauthor, frames the finding more broadly: aging may carry underappreciated benefits that scientists could learn to harness.
Expert Insight
Dr. Lena Morales, a fictional geroscience researcher (University Center for Aging Biology), comments: "These data underscore that aging is not a single, linear decline. Some cellular pathways that change with age may create microenvironments less permissive to tumor initiation. That doesn’t mean aging is protective overall—older adults face more frailty and other illnesses—but it does open a valuable line of inquiry: what protective features of aged tissues can we isolate and translate into therapies?"
Her view highlights a key research challenge: teasing apart beneficial versus detrimental age-associated changes, then determining which are safe to replicate pharmacologically.
Broader implications and next steps
- Model development: Laboratories should incorporate aged animals into preclinical pipelines to capture age-dependent effects on tumor biology and therapy response.
- Mechanistic work: Researchers need to identify which aging signatures—epigenetic marks, metabolic shifts, immune remodeling—drive tumor suppression in old tissues.
- Clinical translation: If aging-linked protective mechanisms are validated, they could inspire drugs that mimic those states in younger patients or enhance therapy in older patients in ways that respect physiological differences.
The study, led by lead author Emily Shuldiner and published Nov. 4 in Nature Aging, represents one of the first controlled demonstrations that aging can actively repress tumor initiation and growth in a mammalian model. It also shows that age-related molecular signatures can persist inside dividing cancer cells, complicating assumptions about how 'young' cancer cells should look at the transcriptome level.
Overall, the work reframes aging in cancer research: rather than viewing age only as an accumulation of damage, scientists should consider it a complex, systemic shift that sometimes places new constraints on malignant cells. Following this thread could reveal novel strategies for prevention and therapy that are tuned to the biology of different age groups.
Source: scitechdaily
Comments
atomwave
Wow, didn't expect aging might block tumors. If they can find the mechanisms and mimic them, huge. But don't assume old = safe, lots else goes wrong.
labcore
Wait, mice get less cancer when super old? sounds wild. Could be real biology or just model quirks, I wanna see more data, strain, immune status etc. hmm

Leave a Comment