4 Minutes
Researchers have identified a specialized class of CD4 immune cells that identify and destroy senescent cells — the so-called "zombie" cells that accumulate with age and drive chronic inflammation. The discovery, led by Ben-Gurion University of the Negev, suggests the immune system adapts to aging by producing targeted killers that keep tissue damage in check.
How the immune system adapts to aging
Cells that enter senescence stop dividing but keep releasing inflammatory molecules that alter their surroundings. Over years this process — cellular senescence — contributes to tissue scarring, organ dysfunction and a chronic low-grade inflammation often called inflammaging. Scientists have long suspected the immune system plays a role in clearing these harmful cells, but the exact players and mechanisms remained unclear.
The new study identifies CD4-Eomes cells, a variant of helper T cells that express the Eomes protein, as a frontline defense against senescent cells. By comparing immune cells from young and old mice, the team found two critical patterns: the presence of senescent cells triggers CD4 T cells to adopt the CD4-Eomes profile, and mice genetically altered to lack that specialization accumulated more senescent cells. In short, CD4-Eomes cells appear to be an adaptive response to the growing senescent burden that comes with age.
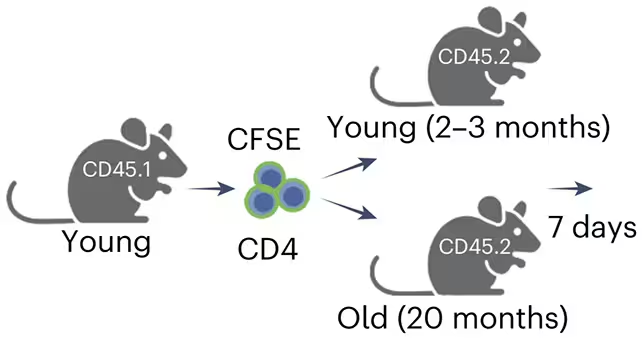
Evidence from experiments and disease models
To test function, researchers transferred CD4 T cells from young animals into both young and aged recipients and tracked the results. Where CD4-Eomes specializations were available, senescent cell numbers dropped and tissue damage markers improved. The protective effect extended to disease models as well: in a mouse model of liver cirrhosis, CD4-Eomes presence limited scarring and reduced levels of senescent cells in damaged liver tissue.
These experiments give direct functional evidence that CD4-Eomes cells help keep senescent cells in check. When those immune specializations were genetically removed, senescent cells proliferated, reinforcing the idea that this immune subset is essential to tissue maintenance as organisms age.
Why this matters for anti-aging research
Many rejuvenation strategies assume that resetting an older immune system back to a youthful state is the key to reversing age-related decline. The Ben-Gurion team’s work cautions against that one-size-fits-all view. An immune profile that includes CD4-Eomes cells may actually be beneficial in older adults, so wiping the slate clean could discard useful, adaptive defenses.
Lead investigators suggest the goal should not be an artificially hyperactive immune system, but one that is properly tuned for a person’s life stage — effective at clearing senescent cells without driving damaging autoimmune-like inflammation.
Next steps and translational prospects
Key questions remain: do human immune systems generate CD4-Eomes cells in the same way, and if so, how do genetics, environment and disease history influence their activity? The research team plans to map CD4-Eomes responses in human tissues and explore whether boosting these cells can safely enhance senescent cell clearance.
If therapies that augment CD4-Eomes function can be developed, they could complement existing approaches such as senolytic drugs that selectively kill senescent cells. But researchers stress caution — translating findings from mice to humans requires careful validation to avoid off-target immune effects.
For now, the discovery frames aging as a dynamic interplay between accumulating cellular damage and immune adaptation, opening a new avenue of research that links cellular senescence, inflammation, and immune specialization.
Source: sciencealert

Leave a Comment