6 Minutes
Breakthrough: enzyme ignites catastrophic DNA shattering in cancer
Researchers at the University of California San Diego have pinpointed the enzyme that triggers chromothripsis — a single, catastrophic event in which a chromosome fragments and is reassembled in a random, destructive order. This form of genome chaos drives rapid tumor evolution, fuels drug resistance, and helps explain why some cancers behave like high-performance machines pushed beyond their design limits.
What the team found
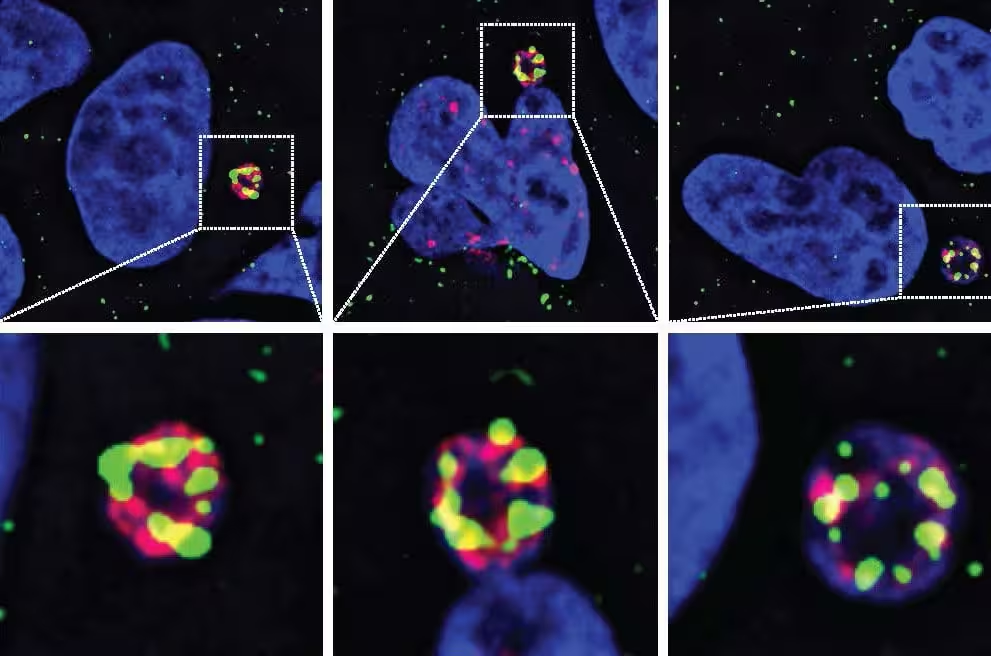
Using live-cell imaging and a systematic screen of known and predicted human nucleases, the UC San Diego group identified N4BP2 as the molecular instigator capable of entering fragile micronuclei and chopping DNA into pieces. When N4BP2 is removed from cancer cells, chromosome shattering dramatically decreases. Conversely, forcing the enzyme into intact nuclei produces widespread DNA breaks even in otherwise healthy cells. The findings were published in Science and resolve a decade-long mystery in cancer genomics.
Why chromothripsis matters
Chromothripsis differs from the slow accumulation of mutations most people imagine. Instead, dozens or hundreds of rearrangements can form in a single episode, accelerating how quickly a tumor gains aggressive traits. Scientists now estimate that roughly one in four human cancers bear the molecular scars of chromothripsis, with especially high prevalence in osteosarcoma and many brain tumors.
Think of it like a car undergoing a massive crash: the chassis is fractured, aftermarket parts get mixed up, and suddenly the vehicle’s performance and handling are unpredictable. In tumors, chromothripsis can create extrachromosomal DNA (ecDNA) — circular, gene-carrying fragments that amplify oncogenes and promote therapy resistance.
How N4BP2 starts the chain reaction
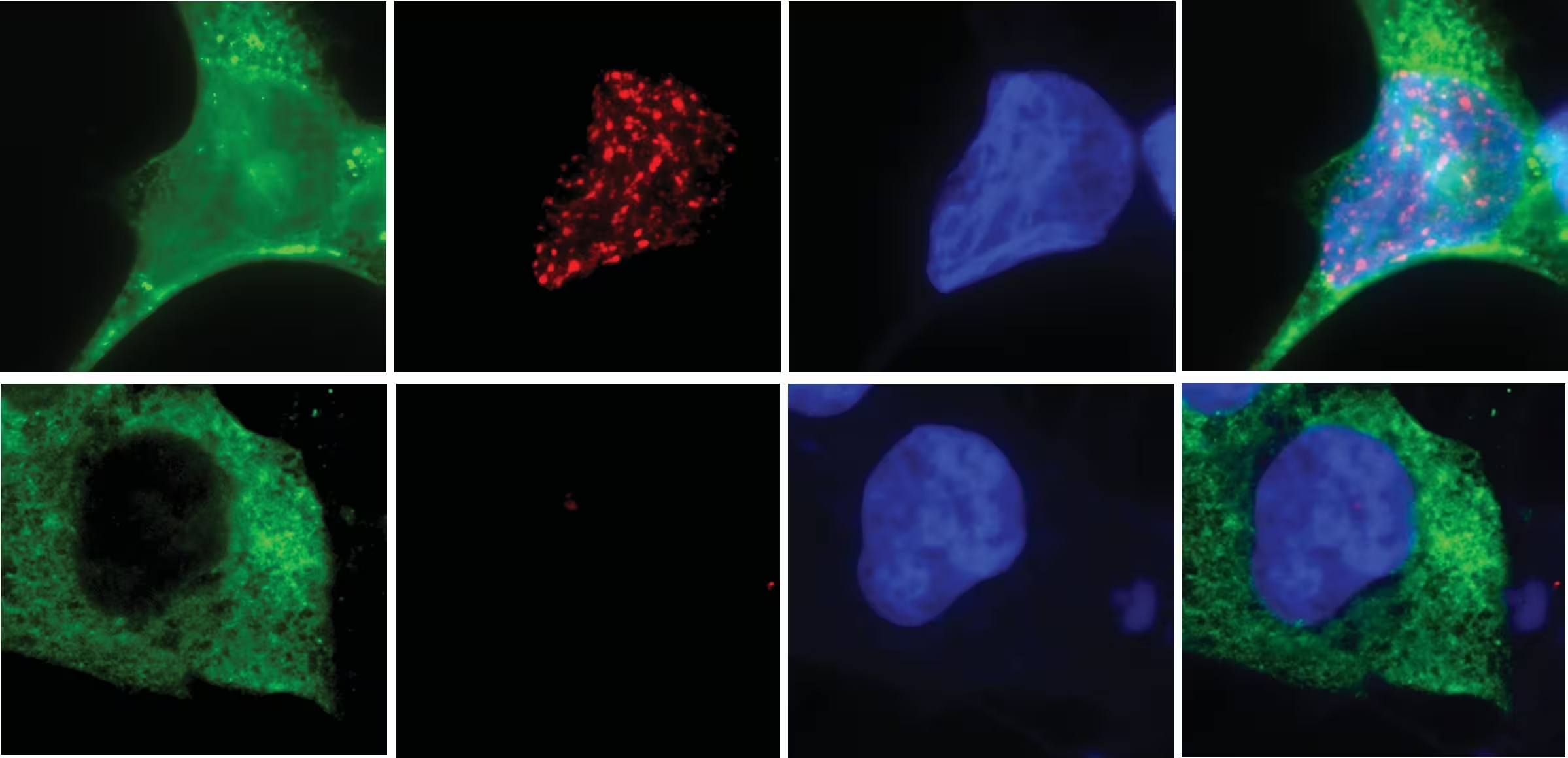
Errors during cell division can strand a whole chromosome inside a tiny, unstable micronucleus. When the micronucleus ruptures, that trapped chromosome becomes exposed to nucleases. While many nucleases exist, the UC San Diego team demonstrated that N4BP2 has the unique ability to penetrate micronuclei and catalyze DNA fragmentation, effectively sparking chromothripsis.
Key experimental observations include:
- Imaging-based screens showed N4BP2 localized to micronuclei where DNA damage accumulates.
- Genetic deletion of N4BP2 in brain tumor cells greatly reduced chromosomal fragmentation.
- Artificially directing N4BP2 into intact nuclei produced DNA breaks, proving sufficiency, not just correlation.
"This enzyme is the missing spark — once you see it, the mechanism of chromothripsis becomes actionable," said Don Cleveland, Ph.D., senior author of the study.
Clinical implications: a new target for aggressive, drug-resistant tumors
Analysis of more than 10,000 cancer genomes linked high N4BP2 expression to increased chromothripsis, structural rearrangements, and elevated ecDNA. Tumors rich in ecDNA are among the most treatment-resistant, and the study reframes ecDNA as a downstream consequence of the broader chromothripsis phenomenon. By positioning N4BP2 at the start of this cascade, researchers have identified a potential molecular handle to reduce genomic instability and slow tumor adaptation.
Potential translational avenues include:
- Small molecules or biologics that inhibit N4BP2 activity or its localization to micronuclei
- Diagnostic assays to measure N4BP2 levels as a biomarker for chromothripsis-prone tumors
- Combination strategies that couple N4BP2 inhibition with existing therapies to prevent rapid emergence of resistant clones
From the lab to the road: automotive analogies and practical perspective
Car enthusiasts often evaluate a vehicle by its engine, chassis, and diagnostics — the same lens helps to conceptualize tumor behavior. N4BP2 functions like a defective ECU (engine control unit) that triggers a cascade, causing critical components to fail simultaneously rather than one at a time. Just as modern OEMs (original equipment manufacturers) use onboard diagnostics to detect and isolate faults early, oncology may now have a molecular readout to flag cancers poised to undergo genomic 'catastrophes.'
For readers who follow automotive trends, this discovery resembles how a new diagnostic sensor can transform maintenance strategies: identifying the fault early prevents a total breakdown. In oncology, targeting N4BP2 could reduce the chance that a tumor suddenly reconfigures itself into a more aggressive, treatment-resistant state — analogous to avoiding a catastrophic engine failure that leaves you stranded.
Industry and research impact
Beyond laboratory intrigue, the finding has implications for clinical development and for how researchers prioritize targets. ecDNA — now connected to chromothripsis through N4BP2 — is a rising focus in cancer research, drawing attention from major funding bodies. Limiting the source of ecDNA formation could change market demand for diagnostics and therapeutics aimed at hard-to-treat cancers such as glioblastoma and osteosarcoma.
For biotech investors and pharma strategists, this offers a clearer target profile: an enzyme that is sufficient to cause catastrophic genomic events and is measurable across thousands of tumor genomes.
Takeaways for general readers and car aficionados
- N4BP2 is the enzyme that can ignite chromothripsis, a rapid and devastating form of genome rearrangement.
- Tumors with high N4BP2 show more chromothripsis and ecDNA, which are linked to treatment resistance.
- Therapies or diagnostics aimed at N4BP2 could help stabilize tumor genomes — much like preventive maintenance stops a mechanical cascade before a total failure.
As with any major discovery, translating this knowledge into safe and effective treatments will take time. Still, identifying the molecular 'spark' changes how scientists think about genome instability and offers a concrete starting point for new anti-cancer strategies.
For car fans who appreciate engineering precision: imagine a future in which doctors can diagnose and neutralize the component that causes catastrophic failure. That is the promise this UC San Diego discovery brings to the fight against the most aggressive cancers.
Source: scitechdaily
Comments
coinpilot
is this even true? sounds huge but could be correlative. Forcing N4BP2 into nuclei made healthy cells break, scary. Off-targets? hope they test that properly
bioNix
wow this N4BP2 finding is wild, like finding the arsonist in a wildfire. If you block it, will tumors actually calm down? hopeful but skeptical... gotta see in patients

Leave a Comment