5 Minutes
Scientists report a promising new candidate drug, NU-9, that appears to slow early Alzheimer’s-related changes in the brain by targeting toxic protein fragments known as amyloid beta oligomers. The discovery—tested in mouse models—highlights a potential preventive strategy that could be given to people at high risk long before memory loss becomes obvious.
Hunting the earliest sparks of Alzheimer’s
For years researchers have suspected that Alzheimer’s disease starts decades before symptoms become visible. Small, soluble assemblies of amyloid beta—called oligomers—are thought to be among the earliest toxic actors, disrupting neurons and triggering inflammation in nearby glial cells. The NU-9 study, led by a team at Northwestern University, focused precisely on those first molecular and cellular shifts that may set the disease in motion.
In their experiments, mice genetically predisposed to develop Alzheimer’s-like pathology were treated with NU-9. The drug reduced levels of amyloid beta oligomers in the brain and, crucially, kept astrocytes in a more quiescent, supportive state. Astrocytes normally maintain neuronal health and regulate synapses, but when they become reactive—a process called astrogliosis—they can amplify neuroinflammation and accelerate damage.
Discovery of a new oligomer subtype and what it means
One surprising outcome from the study was the identification of a previously unrecognized oligomer subtype, labeled ACU193+. The team found ACU193+ forming early inside stressed neurons and binding to astrocytes—potentially serving as a trigger that pushes astrocytes from helper cells into harmful, reactive states.
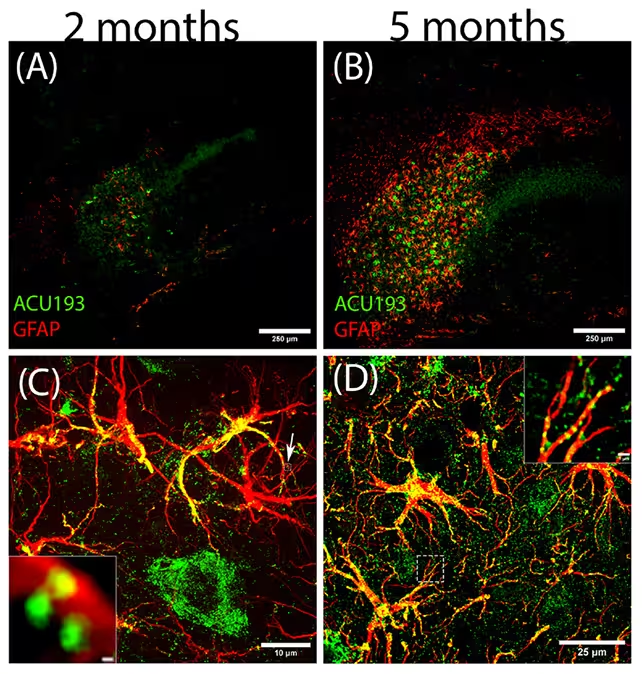
The researchers tracked the growing influence of amyloid beta oligomers (green) and their influence on reactive astrocytes (red).
"These results are stunning," said neurobiologist William Klein, noting the marked effect NU-9 had on reducing reactive astrogliosis, a hallmark of neuroinflammation linked to early Alzheimer's pathology. Northwestern neuroscientist Daniel Kranz added that early molecular events—like intracellular amyloid build-up and glial activation—occur long before clinical symptoms, a mismatch that may explain why many clinical trials fail when they begin too late.
How NU-9 works and why timing matters
NU-9 appears to interfere with the accumulation of amyloid beta oligomers, preventing them from aggregating and interacting destructively with neurons and astrocytes. The drug had previously shown activity against oligomer formation in human brain cells cultivated in the lab; its effectiveness in living animals now strengthens the case for further development.
From animal models to possible human prevention
- Early-stage testing: NU-9 lowered oligomer loads and reduced markers of astrocyte reactivity in mouse models.
- Next steps in animals: Researchers are now testing NU-9 in models that mimic later-stage disease to understand whether the compound can still provide benefit as pathology advances.
- Potential human pathway: If animal data remain encouraging, NU-9 could enter clinical trials aimed at people who show early biomarkers of Alzheimer’s—similar to how statins are used to prevent heart disease in high-risk patients.
It’s important to emphasize that amyloid beta—either as oligomers or plaques—may not be the sole cause of Alzheimer’s. The disease likely arises from multiple interacting factors, including tau pathology, vascular changes, and genetics. Still, targeting a toxic early player like oligomers could be part of a multi-pronged prevention strategy.
Implications for diagnostics and preventative medicine
The prospect of a drug that can be taken before symptoms appear depends heavily on better early detection. New blood-based biomarkers and other diagnostic tools are in development and could identify candidates for preventative therapy. "If someone has a biomarker signaling Alzheimer's disease, then they could start taking NU-9 before symptoms appear," Klein noted, drawing a direct parallel to cholesterol-lowering drugs used to reduce cardiovascular risk.
What researchers are watching next
Key questions remain: Can NU-9 slow or halt progression in later-stage models? Will long-term treatment be safe and effective? And how well will animal findings translate to the human brain’s greater complexity? Ongoing preclinical work aims to answer these questions, while researchers plan the necessary safety and dosing studies required before a human trial can begin.
Expert Insight
"NU-9 represents a promising direction because it intervenes at a molecular stage that typically precedes symptoms," said Dr. Elena Morris, a fictional senior researcher in neurodegenerative disease. "Preventative neurology will require a combination of early detection, well-tolerated therapies, and public-health readiness to treat people who are at risk but not yet sick. NU-9 could be one piece of that puzzle—if larger studies confirm its benefits."
Source: sciencealert
Comments
atomwave
Is this even true? Sounds promising but so many Alzheimer leads flop in humans. What about tau interactions, long term safety and dosing? tbh skeptical
bioNix
Wow this NU-9 news actually gave me chills. If it really quiets astrocytes that's huge, but mice only so fingers crossed... still excited

Leave a Comment