5 Minutes
Researchers at the University of Vermont have identified a surprising molecular brake on blood-vessel pressure sensors in the brain — and restoring that brake could become a new way to treat reduced cerebral blood flow and some forms of dementia. The discovery points to a phospholipid called PIP2 as a potential therapeutic lever to normalize circulation and protect brain function.
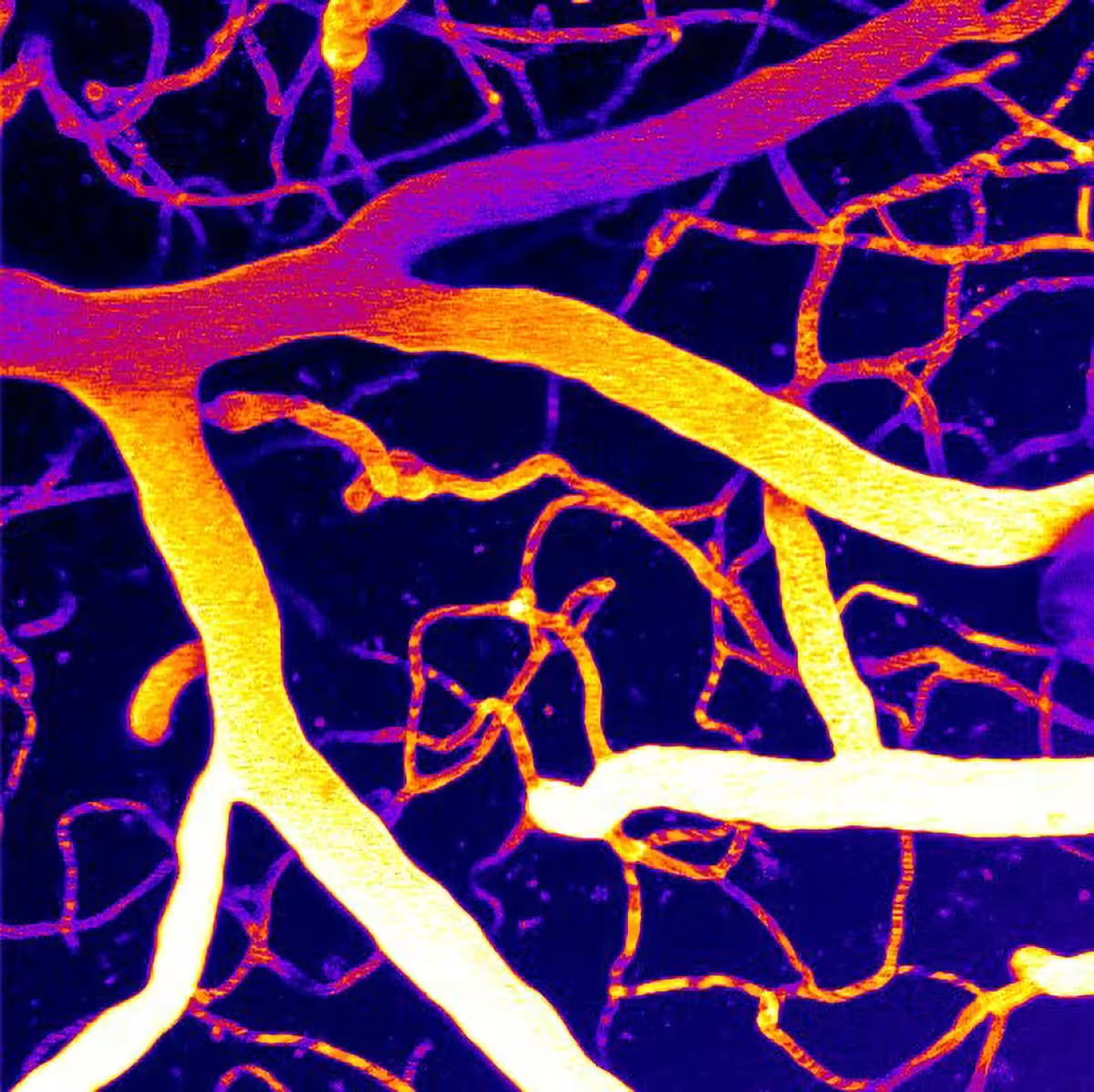
The microvasculature of a brain imaged using two-photon laser scanning microscopy.
How a membrane lipid keeps brain blood flow steady
Maintaining steady blood flow in the brain relies on finely tuned signals between blood, vessel walls, and the surrounding neural tissue. At the center of this control is Piezo1, a pressure-sensing ion channel expressed in vascular cells. Piezo1 opens in response to mechanical forces from flowing blood and helps blood vessels adapt their tone and diameter.
In preclinical work published in Proceedings of the National Academy of Sciences, the team led by Osama Harraz, Ph.D., at the Larner College of Medicine found that activity of Piezo1 can run unchecked when a membrane phospholipid called phosphatidylinositol 4,5-bisphosphate (PIP2) is depleted. PIP2 is known to regulate many ion channels and signal-transduction pathways. In this case, PIP2 acts like a natural inhibitor of Piezo1: when PIP2 levels fall, Piezo1 becomes overactive and disturbs cerebral blood flow.
From lab dish to better circulation: restoring PIP2
Using animal models, Harraz and colleagues tested whether replenishing PIP2 could rein in Piezo1 and restore normal blood flow. Their results showed that restoring PIP2 suppressed excessive Piezo1 activity and helped normalize microvascular circulation in the brain. Importantly, these changes were associated with improvements in brain perfusion metrics that are often impaired in dementia.
"This discovery is a huge step forward in our efforts to prevent dementia and neurovascular diseases," Harraz said. "We are uncovering the complex mechanisms of these devastating conditions, and now we can begin to think about how to translate this biology into therapies."
Why this matters for Alzheimer’s and related dementias
Alzheimer’s disease and related dementias currently affect tens of millions of people worldwide, and vascular dysfunction is increasingly recognized as a major contributor to cognitive decline. Reduced cerebral blood flow, impaired clearance of metabolic waste, and chronic microvascular damage all accelerate neuronal dysfunction.
The UVM work links a mechanistic chain: loss of PIP2 → unchecked Piezo1 activity in brain vessel cells → disrupted cerebral blood flow → potential worsening of dementia symptoms. By targeting either PIP2 levels or Piezo1 function, researchers hope to restore the vascular regulation that keeps brain tissue supplied with oxygen and nutrients.
What’s next: binding sites, membrane physics, and therapy development
Key unanswered questions remain. The Harraz laboratory plans to map exactly how PIP2 restrains Piezo1: does PIP2 bind directly to specific protein regions on the channel, or does it change the surrounding membrane environment so the channel pore is less likely to open? Answering these details will guide strategies for drug development — whether through molecules that mimic PIP2, agents that boost local PIP2 production, or selective Piezo1 inhibitors tuned for brain vasculature.
Future studies will also explore how disease processes reduce PIP2 in the first place. Is PIP2 loss a consequence of inflammation, altered lipid metabolism, or other pathologies common in aging brains? Understanding upstream causes will expand options for intervention and may identify biomarkers to track treatment response in clinical trials.

Osama Harraz, Ph.D., assistant professor of pharmacology at Larner College of Medicine, looks at brain vasculature through a widefield fluorescence microscope in his laboratory at the University of Vermont.
Expert Insight
"Targeting lipid–channel interactions is an emerging frontier in neurovascular medicine," says Dr. Maya Singh, a neuroscientist not involved in the study. "If PIP2 replacement or Piezo1 modulation can be made safe and delivered to the right brain regions, it could complement amyloid- or tau-directed therapies by preserving the vascular support neurons need."
The discovery is an early but promising step. Translating it into human therapies will require detailed mechanistic work, safety testing, and careful design of delivery systems to reach cerebral microvasculature without systemic side effects. Still, by revealing a tangible molecular control point for blood flow, this research opens a new direction for combating vascular contributions to dementia.
Source: scitechdaily
Comments
datacog
Is PIP2 loss cause or effect? If it's inflammation driven, maybe better to target that first. also how do you get lipids into tiny vessels without messing other stuff up?
bioQuill
Whoa, a membrane lipid actually dials down pressure sensors? wild. If they can boost PIP2 in ppl safely, huge — delivery gonna be the hard part tho

Leave a Comment