5 Minutes
New laboratory work suggests we might one day reduce tooth decay and gum disease not by killing oral microbes, but by interrupting the chemical conversations that let harmful species take over. By targeting quorum sensing — the signaling bacteria use to coordinate behavior — researchers show it’s possible to nudge dental plaque toward a healthier mix of microbes.
How bacterial 'chat' shapes your mouth
Bacteria in the mouth don’t act alone. They live in complex communities called biofilms — dental plaque being the most familiar example — and they use chemical signals to sense population density and change gene expression. This process, known as quorum sensing, controls when bacteria produce factors for adhesion, biofilm maturation, or virulence.
One class of signaling molecules, N-acyl homoserine lactones (AHLs), is central to quorum sensing for many gram-negative species. When AHLs reach a threshold concentration, they flip genetic switches that favor late-colonizing species associated with periodontal disease, such as Porphyromonas gingivalis. In contrast, early colonizers like Streptococcus and Actinomyces tend to support oral health.
What the University of Minnesota study found
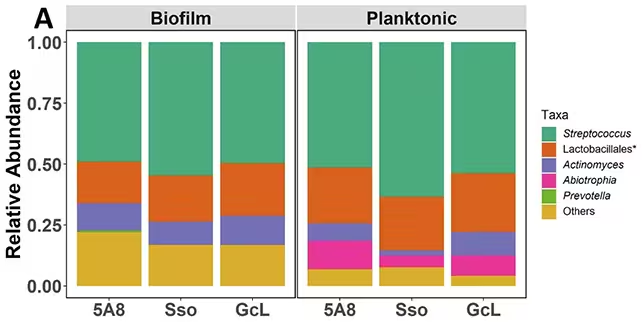
A team led by researchers at the University of Minnesota grew simplified models of dental plaque in the lab to map how AHL-based signaling affects community composition. Instead of relying on broad-spectrum antibiotics, they applied enzymes that interrupt AHL signaling — a strategy called quorum quenching.
The results were striking: blocking AHLs shifted the balance away from disease-associated bacteria and toward health-associated species. Biofilm communities (the surface-attached colonies that make up plaque) were particularly responsive; free-floating, planktonic bacteria showed much smaller changes. The work also revealed important environmental nuances. Bacteria in low-oxygen (anaerobic) niches — like deep pockets below the gumline — may not produce AHLs themselves but can still detect signals produced elsewhere. That cross-talk helps explain how distant microbial populations influence each other in the mouth.
“By disrupting the chemical signals bacteria use to communicate, one could manipulate the plaque community to remain or return to its health-associated stage,” said Mikael Elias, a biochemist involved in the study. Coauthor Rakesh Sikdar added that quorum sensing likely plays distinct roles above and below the gumline, which matters for designing targeted therapies for periodontal disease.
Why this matters for oral and overall health
The prospect of modulating the oral microbiome is attractive because it aims to maintain microbial balance rather than eradicate bacteria indiscriminately. That approach could reduce selection for resistant strains and preserve protective species that help prevent colonization by pathogens.
Oral health ties into broader systemic outcomes: chronic gum disease has been linked to cardiovascular disease, diabetes complications, and even cognitive decline in some studies. If quorum-quenching strategies can be validated in humans, they might provide a precision tool to lower those risks by preventing dysbiosis — the imbalance of microbial communities — in the mouth and possibly in other tissues.
Therapeutic routes and technological possibilities
- Enzymes that degrade AHLs (quorum quenchers) could be formulated as rinses or slow-release gels.
- Probiotic approaches might combine beneficial early colonizers with signal-blocking molecules to favor healthy succession in plaque formation.
- Small-molecule inhibitors of quorum sensing are another avenue, though specificity and safety will be key hurdles.
Limitations and next steps
The study was conducted in simplified lab models, so further research must confirm that these dynamics operate the same way in the human mouth. The researchers did not measure clinical endpoints such as cavity formation or progression of periodontitis. Translating an enzyme or small-molecule quorum quencher into a safe, effective oral treatment will require animal studies, safety testing, controlled human trials, and careful attention to how interventions affect the broader oral microbiome over time.
Expert Insight
“This work is exciting because it reframes treatment as ecological management rather than microbial warfare,” said Dr. Laura Kim, a fictional microbiome scientist at a major research hospital. “Real-world application will hinge on delivery — getting quorum-quenching agents into the right niche without disrupting beneficial microbes — but the concept opens a new path for preventive dentistry and targeted infection control.”
Overall, the study offers a proof of principle that hijacking bacterial communication could be a feasible way to steer oral microbial communities toward health. If future studies support these findings in vivo, dentists and microbiologists may have new, more nuanced tools to prevent tooth decay and periodontal disease while preserving the beneficial members of our oral microbiome.
Source: sciencealert
Comments
Armin
Is this even true? if they make mouthwash that blocks signals, will it wipe out good species too, or just reduce disease? curious but skeptical
microQ
Whoa, bacteria gossiping to cause cavities? mind blown. Hope it works in people tho, lab results often dont translate :/

Leave a Comment