7 Minutes
Researchers in the United States have developed a low-cost, light-driven technique that selectively destroys cancer cells using near-infrared LEDs combined with tin oxide nanoflakes (SnOx). Early lab results show high potency against skin and colorectal cancer cells while sparing healthy tissue — a promising advance for gentler, more accessible cancer therapy.
How light and nanomaterials team up against tumors
Photothermal therapy — using light to generate heat that kills tumor cells — is not new, but this approach rewrites several of its limits. Instead of expensive, highly focused lasers, the new method uses inexpensive near-infrared (NIR) light-emitting diodes (LEDs) together with nanoscopic flakes of oxygenated tin oxide (SnOx). The SnOx nanoflakes absorb NIR light efficiently and convert it into localized heat. When these nanopieces accumulate near malignant cells and are illuminated, they raise the local temperature enough to disrupt cancer cell membranes and proteins, leading to cell death.
The key advantages are practical: LEDs are portable, cheap, and easier to deploy outside high-tech hospital suites. The tin oxide flakes are made through water-based synthesis that avoids toxic solvents, making production more sustainable and compatible with medical applications.
Lab results that caught scientists' attention
In laboratory tests cited by the research team, LED-activated SnOx nanoflakes eliminated up to 92% of skin cancer cells (relevant for melanoma and basal cell carcinoma models) and about 50% of colorectal cancer cells within a 30-minute exposure window. Importantly, normal human skin cells in the same experiments were unaffected, demonstrating a therapeutic window where cancer cells are preferentially damaged.
The reason for this selectivity is twofold: cancer cells often have altered metabolism and greater sensitivity to thermal stress, and localized heating produced by the nanoflakes is confined to the illuminated area. That combination reduces systemic toxicity compared with chemotherapy and lowers risks of collateral tissue damage compared with some laser-based photothermal systems.
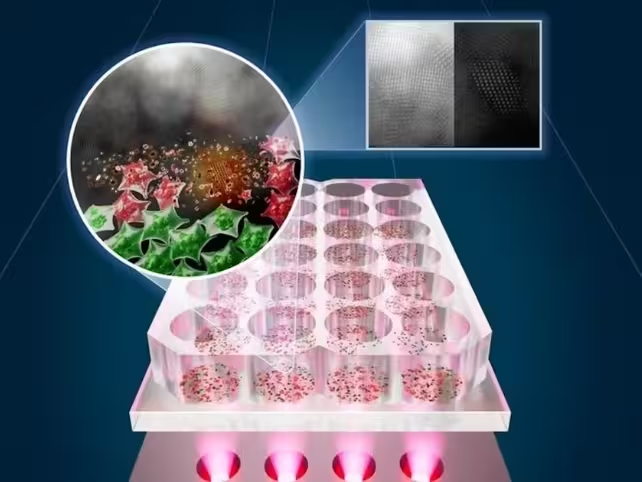
The researchers also provided a visual summary of their setup and results: the custom NIR LED heating system lights up samples where SnOx flakes convert light into heat, shown as a contrast between live (green) and killed (red) cells. (The University of Texas at Austin)
Why SnOx matters: materials science meets medicine
Tin oxide (SnO2 and related oxygenated forms) is already well-known in electronics and transparent conductive coatings. The team transformed tin disulfide (SnS2) into oxygenated tin oxide nanoflakes to boost NIR absorption. This structural change enhances photothermal conversion, meaning a larger fraction of incident NIR energy becomes therapeutic heat.
Because synthesis is water-based and avoids dangerous organic solvents, the process is scalable and more environmentally friendly than many nanoparticle manufacturing routes. Scalability and biocompatibility are important if a technology is to move from benchtop studies into preclinical testing and, eventually, widespread clinical use.
Potential clinical uses and delivery strategies
One immediate application the team envisions is post-surgical sterilization of tumor beds. After removing a melanoma or basal cell carcinoma, clinicians could apply a patch-like LED device with targeted SnOx flakes at the site to eliminate residual malignant cells and reduce recurrence. This patch approach could be compact, affordable, and even adapted for outpatient clinics or remote settings.
Other delivery ideas include topical formulations for superficial tumors, localized injections near resected margins, and eventually implantable microdevices carrying nanoflakes for long-term photothermal control. Researchers are also exploring whether complementary materials or tuned wavelengths might reach deeper tissues for cancers like breast or colorectal tumors.
Combining photothermal therapy with other cancer treatments
Photothermal heating can make tumors more susceptible to immunotherapy and targeted drugs. Mild hyperthermia increases membrane permeability, can expose tumor antigens, and may trigger local immune activation — turning a localized intervention into a systemic advantage when combined with immune checkpoint inhibitors or vaccine strategies.
That means LED-driven SnOx photothermal therapy could be part of combination regimens that reduce drug doses and side effects while preserving or improving therapeutic outcomes. Imagine a scenario where a short LED session after surgery reduces the need for aggressive systemic chemotherapy — a patient-friendly, less toxic path forward.
Safety, accessibility and global implications
Safety is central to the appeal. Unlike chemotherapy, which circulates throughout the body and harms rapidly dividing normal cells, photothermal therapy confines its effect to the illuminated region, producing minimal systemic toxicity and no cumulative organ damage. LEDs produce gentler, more uniform heating than intense lasers, decreasing the risk of burns and collateral tissue injury.
Because LEDs and water-based SnOx synthesis are inexpensive and portable, the technology could extend advanced localized cancer care to low-resource regions that lack expensive radiotherapy or laser infrastructure. Early detection of superficial tumors could be treated outside major hospitals, improving access and reducing healthcare costs.
Expert Insight
“This approach addresses several practical barriers at once,” says Dr. Emily Vargas, a fictional biomedical engineer specializing in photonic medical devices. “Using near-infrared LEDs lowers equipment and operational complexity while the SnOx flakes provide strong photothermal conversion with a production route that’s compatible with clinical standards. If preclinical safety and biodistribution profiles hold up, this could become an affordable adjuvant for surgical oncology.”
Another imagined clinician, surgical oncologist Dr. Marcus Lee, adds: “A patchable LED system that sterilizes margins could reduce re-excision rates and patient anxiety after melanoma surgery. The simplicity matters — patients and clinics are more likely to adopt a technology they can apply quickly and safely.”
Next steps and research challenges
Translation into human trials requires several milestones: detailed preclinical safety studies; clarification of how SnOx flakes distribute, metabolize, and clear from tissues; and optimization of LED wavelengths, intensities, and exposure times for different tumor types. Researchers are also testing whether alternative or complementary nanomaterials could extend penetration depth for internal tumors.
There are open questions about immune effects, long-term biocompatibility, and how best to integrate photothermal protocols with standard-of-care therapies. Still, the simplicity of LED-based systems reduces barriers to iteration and rapid testing, making this an attractive line of inquiry for both academic and translational teams.
Light is one of nature’s simplest energies, and harnessing it with affordable LEDs and engineered nanomaterials could reshape localized cancer care. If follow-up studies confirm safety and efficacy, LED-activated SnOx photothermal therapy may offer a precise, less toxic, and more accessible option for treating certain cancers — from skin lesions treated in a clinic to combined protocols with immunotherapy that amplify the body’s own defenses.
Source: sciencealert
Comments
skyspin
is this even true? 50% for colorectal seems low, and what about SnOx clearance and long term effects... penetration depth with NIR LEDs? not sold yet
labcore
Wow this is wild, LEDs plus SnOx doing the job? If preclinical safety checks out, could be a game changer for clinics with less tech. hopeful but cautious

Leave a Comment