5 Minutes
Researchers have shown that fixing the activity balance in a very small group of neurons in the amygdala can erase anxiety and social withdrawal in mice. The result points to an unexpectedly precise brain mechanism that could guide future, circuit-targeted treatments for affective disorders.
Researchers found that rebalancing activity in a tiny amygdala circuit was enough to reverse anxiety and social withdrawal in mice. The discovery highlights a precise brain mechanism that could inspire more targeted mental health treatments.
How a single circuit tipped the emotional scale
The study, led by Juan Lerma's Synaptic Physiology laboratory at the Institute for Neurosciences (IN) — a collaboration between the Spanish National Research Council (CSIC) and Miguel Hernández University (UMH) of Elche — traced anxiety and social deficits to a discrete population of neurons in the basolateral amygdala. By restoring normal excitability within that circuit, the team reversed behavioral symptoms in mice that closely resembled aspects of human anxiety, depression, and social withdrawal.
"We already knew the amygdala plays a central role in fear and anxiety, but this work shows that the unbalanced activity of a specific cell population is by itself sufficient to produce pathological behaviors," Lerma said. The findings were published in iScience and open a window onto how tiny shifts in neural signaling can reshape emotional states.
Genetic engineering to create and then correct imbalance
To reveal the mechanism, the researchers used a genetically modified mouse line that overexpresses the Grik4 gene. That change increases GluK4-type kainate receptors on certain neurons, making them hyperexcitable. These Grik4-overexpressing mice display marked anxiety and reduced social interaction — phenotypes that the team had first characterized in 2015.
Using viral vectors and molecular tools, the group then selectively corrected Grik4 expression in neurons of the basolateral amygdala. That localized intervention re-established normal communication with a downstream group of inhibitory cells in the centrolateral amygdala — the so-called 'regular firing neurons' — restoring a balanced excitatory–inhibitory relationship across the microcircuit.
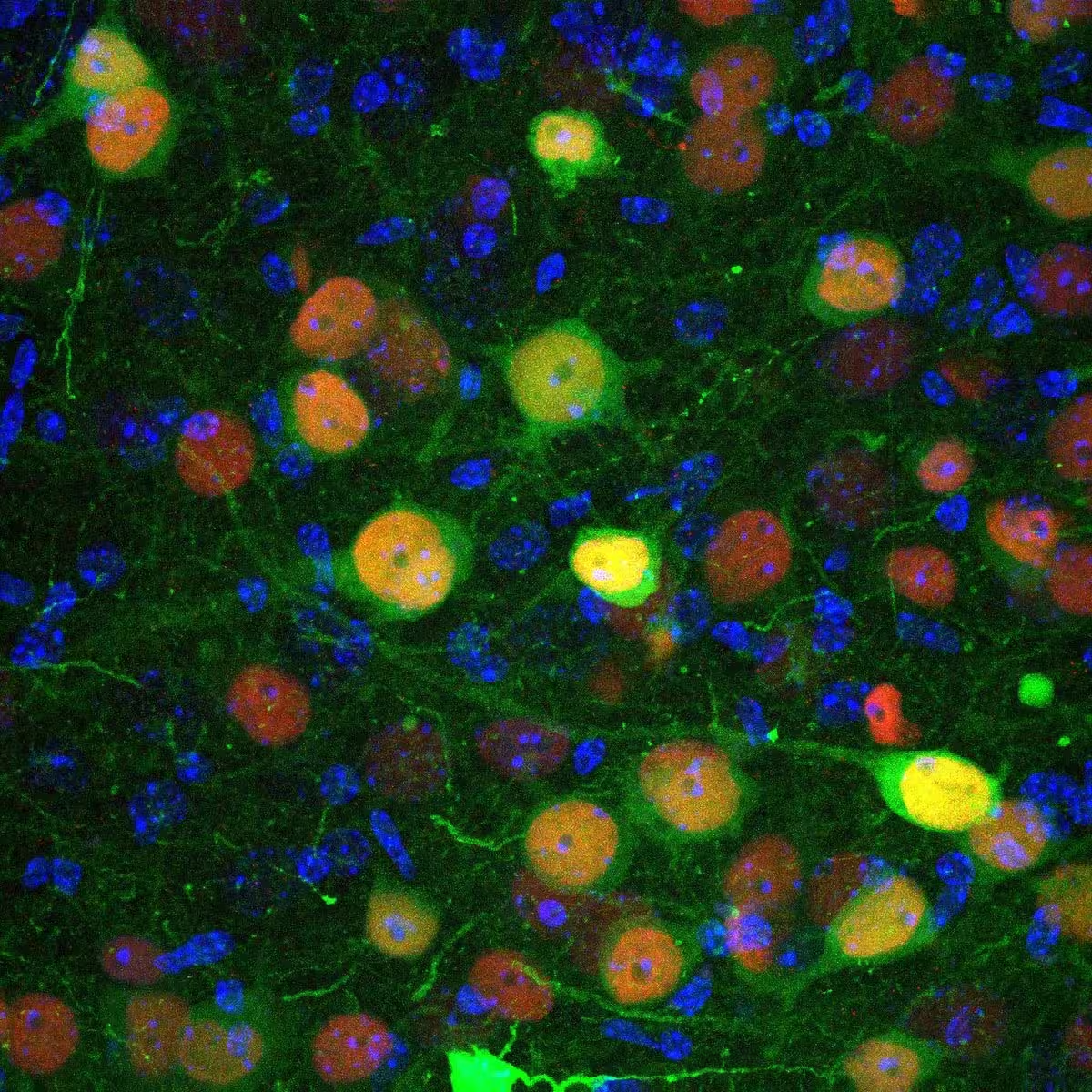
Confocal microscopy image showing basolateral amygdala cells infected by a virus engineered to introduce the CRE recombinase protein (in red) and the fluorescent protein GFP (in green), allowing visualization of the infection.
Measuring circuits and behavior in tandem
The team combined electrophysiological recordings with a battery of behavioral assays to link synaptic changes to mouse behavior. Neural recordings confirmed that correcting Grik4 expression normalized firing rates and synaptic responses in the basolateral-centrolateral amygdala pathway. Behaviorally, the treated mice explored open spaces more readily and showed restored interest in unfamiliar conspecifics — standard measures for reduced anxiety and improved social motivation in rodents.
First author Álvaro García emphasized the elegance of the result: 'That focused adjustment in one amygdala microcircuit was enough to reverse anxiety-like and social deficit behaviors — a remarkably specific rescue.' Genetic targeting and localized viral repair allowed the group to test causality at circuit resolution, rather than relying on broad pharmacology.
Not a universal fix, but a promising blueprint
Importantly, the researchers tested the same approach in non-modified, wild-type mice that naturally exhibited higher anxiety. The intervention also reduced anxiety in these animals, suggesting the mechanism may generalize beyond the Grik4 genetic model. "This gives confidence that the circuit principle we uncovered could apply more broadly to how emotions are regulated," Lerma noted.
However, some deficits were not rescued. Treated animals still had impairments in object recognition memory, indicating that cognitive symptoms involve additional regions such as the hippocampus. The team is careful to frame the finding as a targeted strategy rather than a single cure: correcting a defined amygdala microcircuit mitigates specific affective symptoms but does not erase all behavioral consequences of the underlying genetic alteration.

IN CSIC-UMH researchers Álvaro García, Juan Lerma, Ana Valero Paternain, and María Isabel Aller.
Why this matters for future therapies
The study highlights several translationally relevant ideas: first, that affective symptoms can arise from local imbalances in excitability and synaptic connectivity; second, that precise molecular targets (like Grik4/GluK4 signaling) can be modulated in a restricted brain region to produce behavioral benefit; and third, that circuit-level interventions — whether genetic, pharmacological, or neuromodulatory — may offer more focused alternatives to systemic drugs.
Looking ahead, therapies inspired by this work could aim to rebalance excitatory and inhibitory dynamics in defined amygdala microcircuits using targeted delivery systems or refined neuromodulation. But translating mouse circuit discoveries into safe human treatments will require mapping comparable cell types in the human amygdala and developing delivery methods that meet clinical safety standards.
Expert Insight
Dr. Elena Ruiz, a clinical neuroscientist not involved in the study, commented: 'This research is exciting because it links molecular genetics to circuit physiology and behavior in a causal chain. It doesn't promise an immediate therapy, but it does show that highly specific circuit corrections can have major behavioral effects — that is the kind of mechanistic clarity we need to design next-generation treatments.'
Source: scitechdaily
Comments
Armin
Is this even true? fixing a few neurons and voila? sounds promising but feels oversimplified, human brains messy tho, needs loads more work
bioNix
Wow tiny circuit fixing anxiety? mind blown 😮 feels sci fi but gives hope, curious how it'll map to humans, fast

Leave a Comment