5 Minutes
A new injectable nanomaterial shows promise for protecting brain tissue after ischemic stroke by limiting the collateral damage that can follow restoration of blood flow. In mouse experiments the therapy reduced inflammation, curtailed immune-driven injury and supported neural repair — suggesting a potential adjunct treatment to reperfusion therapies.
How a 'dancing molecule' works where others struggle
Researchers at Northwestern University have developed IKVAV-PA, a regenerative nanomaterial built from supramolecular therapeutic peptides (STPs). These STPs — nicknamed 'dancing molecules' because their molecular components move and reassemble dynamically — are engineered to interact with cells in flexible ways. That adaptability appears to help them target damaged brain tissue and encourage nerve cells to repair themselves after an ischemic event.
Ischemic stroke, the most common type of stroke, happens when a blood clot blocks an artery to the brain. Rapidly restoring blood flow is lifesaving, but reperfusion can release toxic molecules into circulation, causing secondary inflammation and further injury. The new peptide material is designed to blunt that harmful cascade while promoting tissue recovery.
Systemic delivery: a practical route to the brain
A crucial advance in this study is the systemic route of administration: the peptides are injected into the bloodstream rather than delivered directly into brain tissue. Systemic delivery is faster and less invasive, and the team demonstrated that the peptides cross the blood-brain barrier to reach the stroke site. In treated mice, the therapy produced minimal off-target disruption, indicating selective action at injured brain regions.
In lab tests the mice receiving the IKVAV-PA injection following reperfusion showed reduced areas of tissue damage, lower markers of inflammation and fewer signs of damaging immune responses than untreated controls. In short, the treatment both dampened the inflammatory aftermath of clot removal and appeared to encourage neural network preservation or repair.
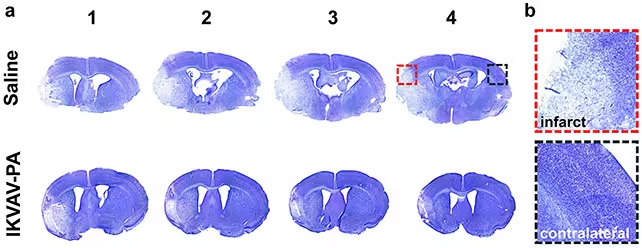
Brains in the treated mice (bottom row) showed fewer signs of brain tissue damage (the lighter-shaded regions).
Scientific background and mechanism
Supramolecular peptides self-assemble into nanoscale structures that can present biological signals to cells in a controlled way. IKVAV-PA carries motifs that encourage neuron adhesion and growth while also imparting anti-inflammatory effects. When reperfusion releases a surge of harmful molecules, those anti-inflammatory properties can reduce secondary cell death and break the feedback loop that amplifies injury.
Materials scientist Samuel Stupp, who led the work, highlights two benefits: systemic administration and blood-brain barrier permeability. Together these features could extend the approach beyond stroke to other disorders where delivery to the central nervous system is a barrier, including traumatic brain injury and certain neurodegenerative conditions.
Implications for patients and next steps
The mouse results are encouraging, but translating a lab success into a clinical therapy requires cautious, long-term study. Safety over extended periods, dosing windows relative to reperfusion, and the potential for immune reactions in humans all need careful evaluation. If those hurdles are overcome, the peptide could become a secondary treatment given alongside established reperfusion techniques to reduce disability after stroke.
Neuroscientist Ayush Batra notes that any therapy reducing long-term disability would carry enormous personal and societal benefits, decreasing the emotional and financial burden on patients, families and healthcare systems. Tens of millions of people worldwide survive strokes each year, but many suffer chronic impairment that interventions like IKVAV-PA hope to lessen.
Related technologies and future prospects
This research sits at the intersection of biomaterials, neurology and immunology. Other groups are exploring similar peptide-based scaffolds, nanoparticle carriers and engineered proteins to modulate inflammation and promote repair. A systemic peptide that reliably reaches injured brain tissue could become a platform: swapping or combining functional motifs might tailor treatments for spinal cord injury, ALS or targeted neuroregeneration.
Real-world application will depend on human trials. Researchers will need to establish optimal timing relative to clot removal, potential interactions with clot-dissolving or mechanical thrombectomy procedures, and long-term outcomes such as functional recovery and risk of recurrent events.
Expert Insight
Dr. Lena Morales, a neurologist and translational research specialist, comments: 'The idea of a blood-borne material that homes to injured brain tissue and both calms inflammation and supports neurons is compelling. In practice, the window for safe delivery after reperfusion will be crucial. If future studies confirm consistent targeting and low systemic toxicity, this approach could change how we think about acute stroke care.'
For now, IKVAV-PA offers a promising proof of concept: dancing molecules that might one day dance patients back toward better recovery.
Source: sciencealert
Comments
Tomas
Solid concept tbh. If it reliably homes to injury, rehab times could drop a lot. timing will be key, tho
labcore
Wait, systemic peptides crossing BBB? Sounds promising but kinda scary. What about long term immune reactions, dosing windows, ppl variability if that's real then...

Leave a Comment