3 Minutes
Microbes behave differently in orbit. New experiments on the International Space Station reveal that bacteriophages — viruses that infect bacteria — and their bacterial hosts evolve along altered paths in microgravity, producing changes that could help fight antibiotic-resistant infections on Earth.
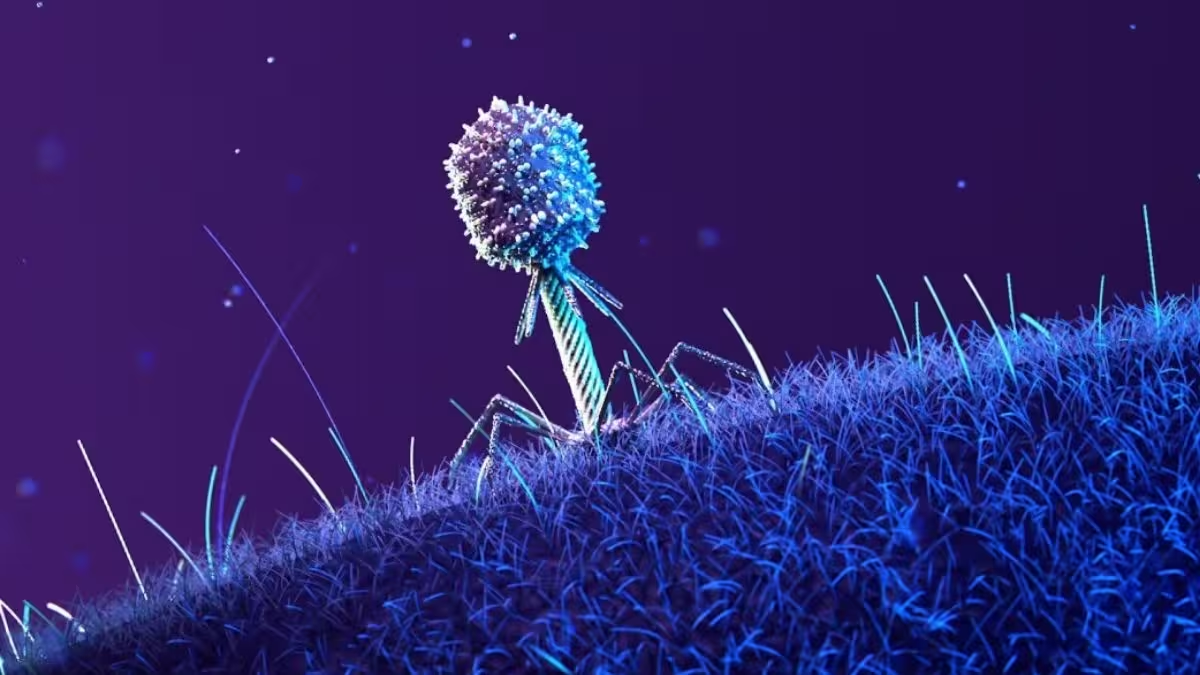
A phage rests on the surface of a host cell.
Why biology in microgravity matters
Imagine an evolutionary arms race happening inside a sealed box on the International Space Station (ISS). That’s effectively what researchers from the University of Wisconsin–Madison and biotech firm Rhodium Scientific did when they sent a mixed population of Escherichia coli bacteria and their viral predator, the T7 bacteriophage, into orbit in 2020. The same experiments ran in parallel on Earth for 25 days so scientists could directly compare how weightlessness shifts microbial evolution.
How the experiment was run
Aboard the ISS, astronauts incubated different combinations of bacteria and phages under controlled conditions. On Earth, a team led by biochemist Vatsan Raman repeated the protocols in Madison. By sequencing genomes and tracking infection dynamics, researchers could see which mutations appeared in space-grown microbes and how those changes affected infection and killing rates when returned to Earth.
Surprising genetic changes and new vulnerabilities
The space environment slowed initial infections and pushed both organisms down evolutionary routes not seen in ground controls. E. coli adapted by mutating genes tied to stress response, nutrient uptake and by altering surface proteins — the very receptors phages latch onto. After a delayed start, the T7 phages evolved new mutations that restored their ability to recognize and bind these modified bacterial surfaces.
Crucially, several of the phage mutations that arose in microgravity proved exceptionally effective at killing antibiotic-resistant strains of bacteria that cause urinary tract infections (UTIs). With more than 90% of UTI-causing strains showing resistance to standard antibiotics in some settings, phage therapy is increasingly studied as an alternative or complement to traditional drugs.
What this means for phage therapy and antibiotic resistance
"Space fundamentally changes how phages and bacteria interact," the researchers note, adding that the space-driven adaptations provided biological insights useful for engineering phages with improved activity against drug-resistant pathogens on Earth. In practice, that means mutations observed in orbit could guide lab design of phage treatments targeted at specific, resistant bacterial strains.
Beyond UTIs, these findings highlight the value of studying evolution under atypical physical conditions. Microgravity is a unique selective pressure that can reveal genetic pathways and molecular interactions otherwise hidden in terrestrial experiments. For researchers designing next-generation antimicrobials, that’s a promising new toolkit.
Future prospects: targeted phages and space biology
Follow-up work will aim to characterize mechanisms behind the most potent space-evolved phage variants and test safety and efficacy in preclinical models. The study underscores a broader point: space biology doesn’t just inform astronaut health — it can accelerate discovery of novel solutions to urgent public-health problems such as antibiotic resistance.
Source: scitechdaily

Leave a Comment