5 Minutes
Pancreatic cancer doesn’t wait. It gathers allies in the tissue long before a detectable tumor appears, and one of its earliest recruits is surprising: the nervous system. Researchers at Cold Spring Harbor Laboratory report that nerve fibers and specialized fibroblasts form a cooperative network that helps pre-cancerous lesions evolve into malignant disease.
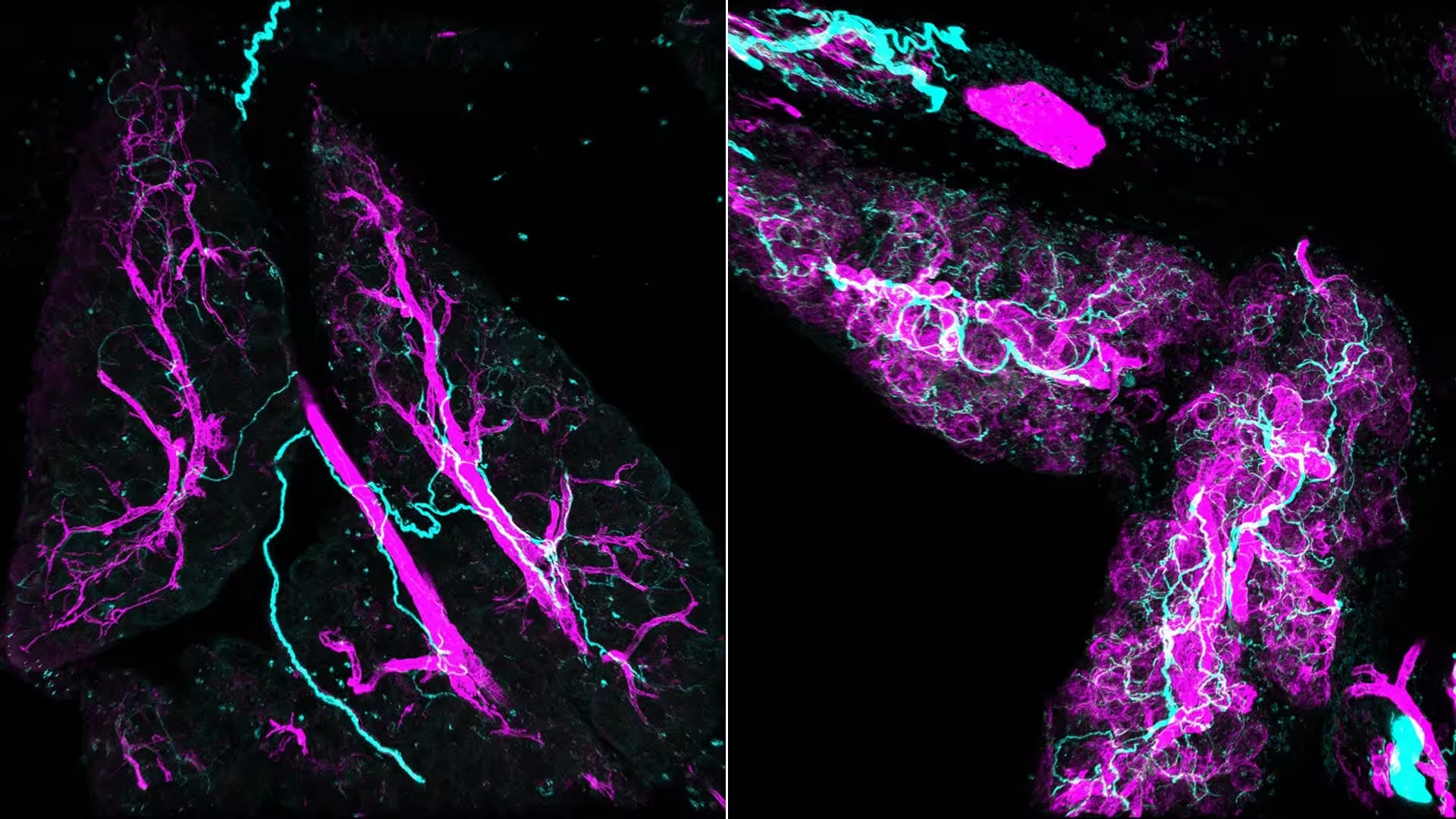
Stress signals redesign tissues during inflammation and cancer. Left: Normal tissue maintenance. Right: Stress signals “switch on” fibroblasts, turning them into myCAFs (purple) that actively recruit nerves (cyan) into the pancreas.
Think of the pancreas like a quiet neighborhood. Then a few houses change—cells begin to misbehave. Instead of an immediate siege, the neighborhood remodels itself: support cells called fibroblasts shift into an activated state, immune signaling changes, and nerve fibers extend into the area. The outcome is a remodeled microenvironment that favors malignant transformation. It’s not simply cancer cells taking over; it’s a covert collaboration.
Seeing the unseen with 3D imaging
Traditional microscopy flattens complex tissues into thin slices. That makes long nerve tracts look like scattered dots. The team used whole-mount immunofluorescence to map lesions in three dimensions, and the view was revelatory: dense, braided nerve bundles weaving through and around activated fibroblasts—myofibroblastic cancer-associated fibroblasts, or myCAFs. When the image appeared, the researchers realized how much of the early microenvironment had been invisible to standard techniques.
The 3D reconstructions don’t just look striking; they change the story. Nerves aren’t passive cables that cancer later exploits. They are early participants, drawn in by chemical cues from myCAFs and then feeding back signals that further activate those same fibroblasts. This two-way crosstalk establishes a self-reinforcing loop that promotes cellular behaviors associated with tumor growth.
One secret weapon in that loop is norepinephrine, a neurotransmitter released by sympathetic nerve fibers. When norepinephrine reaches fibroblasts it triggers calcium signaling inside the cells, a rapid biochemical change that boosts their pro-tumor functions and encourages further nerve growth. The result: a progressively entrenched niche that supports malignant change.
Mechanism and therapeutic implications
Mouse models and human cell experiments both support the same sequence: myCAFs emit attractant signals; sympathetic nerves extend into the lesion; nerves release norepinephrine; fibroblasts respond and become more active; the lesion advances. Disrupt any link in this chain, and the disease falters. In laboratory tests, chemical disruption of sympathetic activity—either by neurotoxins or by blocking adrenergic signaling—reduced fibroblast activation and slowed tumor growth, in one report approaching a nearly 50% reduction in tumor size in affected models.
That finding opens practical doors. Drugs already approved for unrelated conditions, such as adrenergic blockers like doxazosin, may be repurposed to interfere with nerve-driven microenvironment remodeling. Paired with chemotherapy or immune therapy, such agents could blunt progression during a window when the disease is still localized and more vulnerable. Clinical translation will require careful trials, but the advantage is clear: attacking the ecosystem that helps cancer establish itself rather than only targeting the cancer cells.
Beyond therapeutics, the study underscores a diagnostic implication: nerve remodeling might serve as an early biomarker. If clinicians could detect or image this neural infiltration reliably, they might identify at-risk tissue sooner—and intervene before tumors consolidate.
Expert Insight
"We used to think nerves mattered mostly for pain or for late-stage invasion," says a senior researcher involved in the work. "This study flips that view: nerves are active architects of the tumor microenvironment from the beginning. Blocking their signals could be a preventative strategy, not just a palliative one."
A focused effort will be needed to translate these findings into human trials. Researchers will have to define which signaling molecules are most critical, whether blocking sympathetic activity affects normal tissue function, and how best to combine neural-targeted drugs with existing therapies. Funders and patient advocacy groups have already expressed interest, recognizing that new angles may be essential to improve outcomes in a cancer notorious for late detection and poor response rates.
There remains a larger lesson: tumors are ecosystems. They recruit, persuade, and co-opt surrounding cells and systems—including the nervous system itself. Detect the recruitment early, and you may cut the conversation short.
Source: scitechdaily
Comments
atomwave
Nerves recruiting fibroblasts? sounds intense. But is this just mouse model hype, or real human tissue timing? Need solid imaging in ppl
bioNix
wow didn't expect nerves to be early partners in cancer, that's wild. If blocking adrenergic signals helps, huge deal... but cautious.

Leave a Comment